Case study
Cleaner than the original: mAb biosimilar without problematic protease
"We can use the HCP impurity data for both regulatory filing and marketing material"
In this project, a biopharma company needed to compare the impurity profile of their mAb biosimilar to that of the originator product. Interestingly, SWATH LC-MS analysis identified several host cell proteins (HCPs) in the originator drug product batches that were not found in the biosimilar.
The client is a multinational biopharmaceutical company focusing on developing best-in-class biosimilar medicines. Their own observations showed a difference in drug stability when comparing the originator to their biosimilar, where the latter was proving to be more stable. Therefore, they came to us with a particular interest in investigating the impurity profile to explain the stability differences.
Why you should look for problematic HCPs
After our analysis, especially one HCP, identified in the originator, caught our eye. Cathepsin is a protease that may decrease the stability of the therapeutic protein. This specific protease belongs to a group of enzymes known to cleave various recombinant proteins, such as antibody fragments, monoclonal antibodies, bispecific antibodies, and fusion proteins.
Therefore, cathepsin is considered potentially problematic, and you should identify and remove it to increase the shelf life of your product. Moreover, increased stability is a safety issue and often affects the Cost of Goods Sold (COGS). Thus, better stability may result in significant economic savings during the life span of the drug product.
No problematic HCPs detected in the client’s biosimilar
We also analyzed the biosimilar product by mass spectrometry, and the comparison results are in the table below. Interestingly, we only detected cathepsin in the originator and provided our client with data showing that their product did not contain any detectable HCPs known to be problematic. This knowledge thus supplied them with extra ammunition for regulatory documentation. Also, they now had further sales arguments on why you should choose their product over the originator.
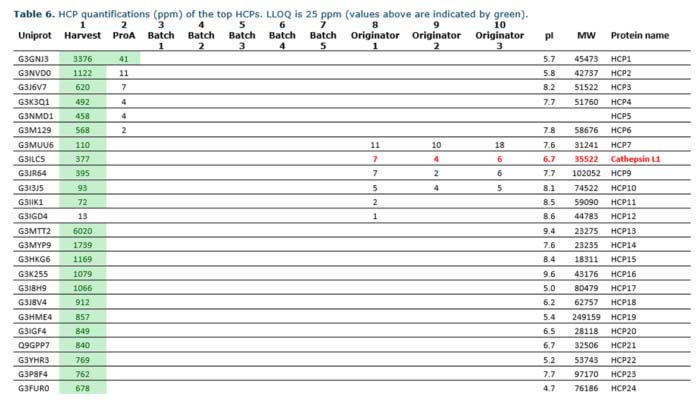
Mass spectrometry results from analyzing a biosimilar and its originator product. The protease, cathepsin, was detected in all three batches of the originator but not in the biosimilar DS batches.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.