Case Study
Analyzing heterogeneous HCP mix from multiple species
"We went from inconsistent ELISAs to reproducible & detailed HCP analysis"
A biopharma developing gene therapies used commercial and custom HCP ELISAs for analyzing Host Cell Proteins during early process development. However, they discovered that the antibodies had low coverage of the product’s HCPs and the measured levels were inconsistent.
Thus, they decided to support their HCP analysis with an orthogonal method. The client selected one that works great in combination with ELISA results or as the only HCP method: Liquid Chromatography Mass spectrometry (LC-MS).
This particular European biopharma company develops highly advanced immunotherapies for cancer patients. They specialize in immune activators that target hard-to-treat solid tumors.
After a referral from their CMO, which had already collaborated with Alphalyse on several projects, the company reached out. Previously, they used commercial and process-specific ELISA kits for HCP analysis. However, they found that they were not reproducible and consistent in the determined HCP amounts.
They suspected that it might be because of their heterogeneous HCP mix, with HCPs from multiple species. The HCPs originated from the cell line used to produce the adenovirus proteins that constitute the drug product, enzymes added in the process, proteins from the cell media, and common contaminants such as human keratins.
They were thus looking into MS methods that could search for all these proteins in one assay. And what they found was a method that could even quantify the adenovirus proteins while documenting HCPs.
Easy comparison of impurity levels in different batches
In particular, they were looking for a more robust and reproducible analysis for the total HCP amount in different batch samples, along with detailed information about individual HCPs. Once they received the results, they could easily compare impurity levels between different batches and optimize their processes to more efficiently reduce HCP levels.
Here the LC-MS results are compared to the client’s ELISA results. It’s important to note that the two methods are two distinct ways of measuring HCPs and that the resulting numbers are not directly comparable.
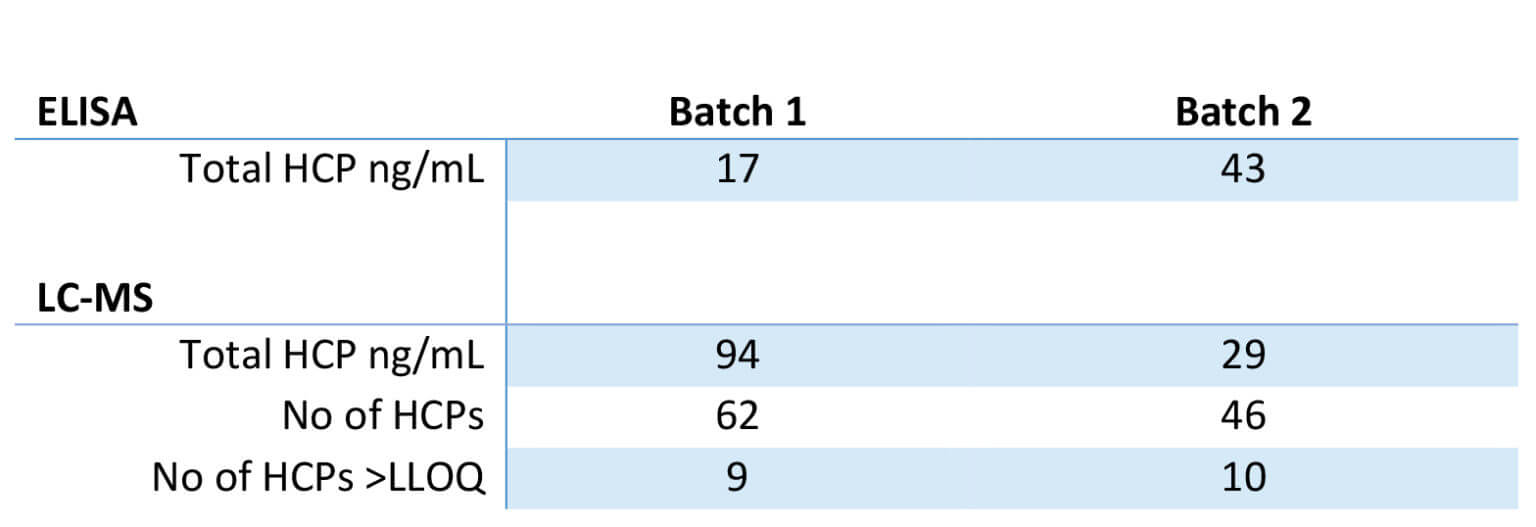
With LC-MS, the client got a detailed list of individual HCPs and their amount in ng protein/mL sample:
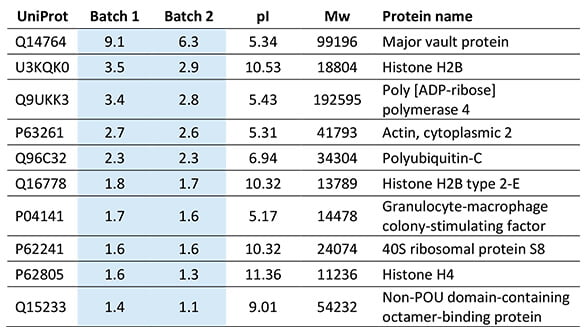
List of Top 10 most abundant host cell proteins in the two adenovirus product batches. The protein amount is in ng/mL.
Bonus: Quantifying viral proteins along with HCPs
Even though it was not a part of the client’s project aim, it was also possible to quantify the adenovirus proteins in the two samples. After seeing the results, they now rely on the LC-MS method for both viral protein quantification and evaluation of their ELISA with the orthogonal HCP quantification.
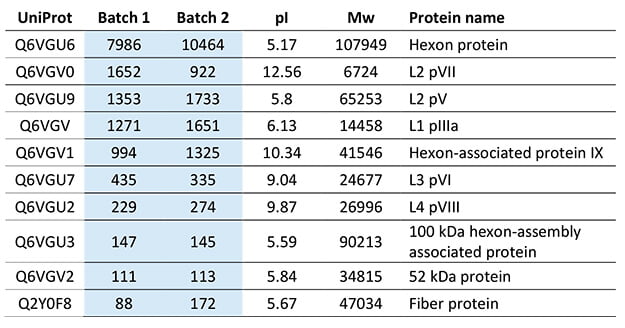
Adenovirus quantification of the top 10 most abundant proteins, listed in ng/mL.
Lesson learned: Never rely on just one method to analyze process-related impurities – and get viral protein quantification results without setting up an additional assay.
Find out more about the gene therapy product analysis here
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.