Case study
Documenting product quality and process consistency
"The most sensitive and robust HCP analysis we’ve seen"
This client uses HCP analysis by mass spectrometry on an ongoing basis to optimize their process development, analyze product quality, and document residual proteins.
As a result, they stay in the frontline of inhalation therapy.
The North American-based company develops novel inhalation therapies for the treatment of patients with rare pulmonary conditions. The lead products are in phase 3 development with a planned market release within a year.
In short, the analytical services performed by Alphalyse include detailed characterization of biologic samples by intact mass analysis and peptide characterization.
- Additional focus is on the downstream process for batch-to-batch comparability and also process optimization.
- Specifically, process-related impurities have been identified and quantified through their purification process with the Alphalyse HCP analysis using SWATH mass spectrometry technology.
The overview below shows the six-step purification process of the client’s recombinant protein:
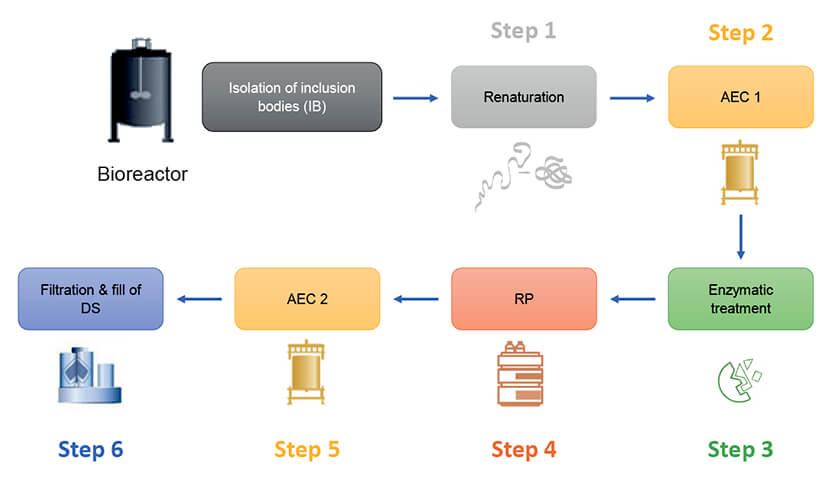
Process Performance Qualification
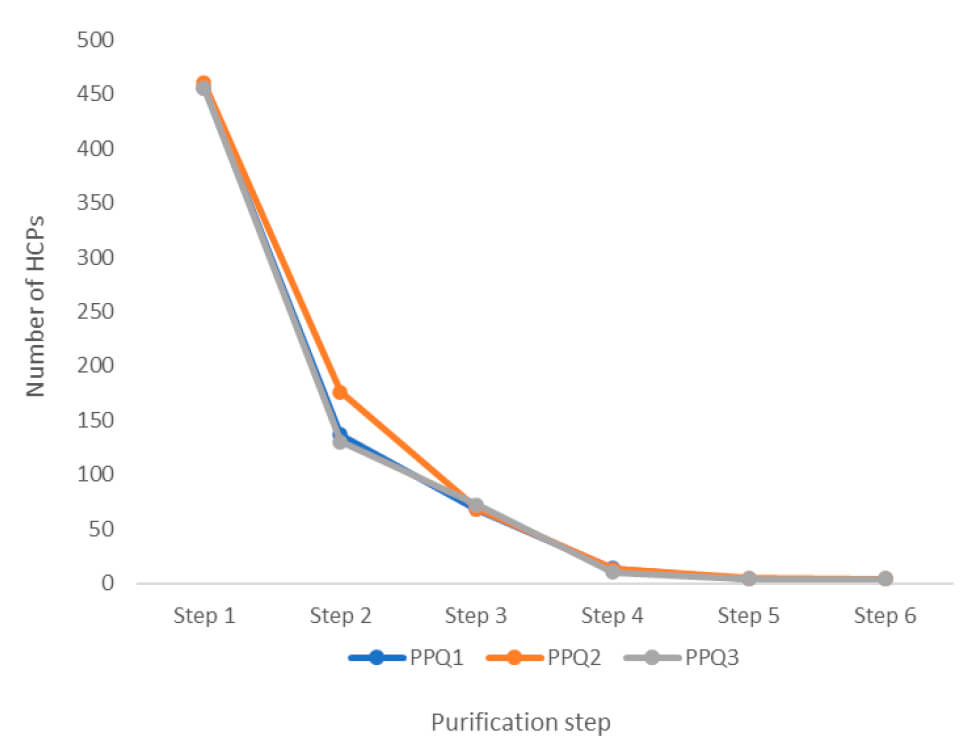
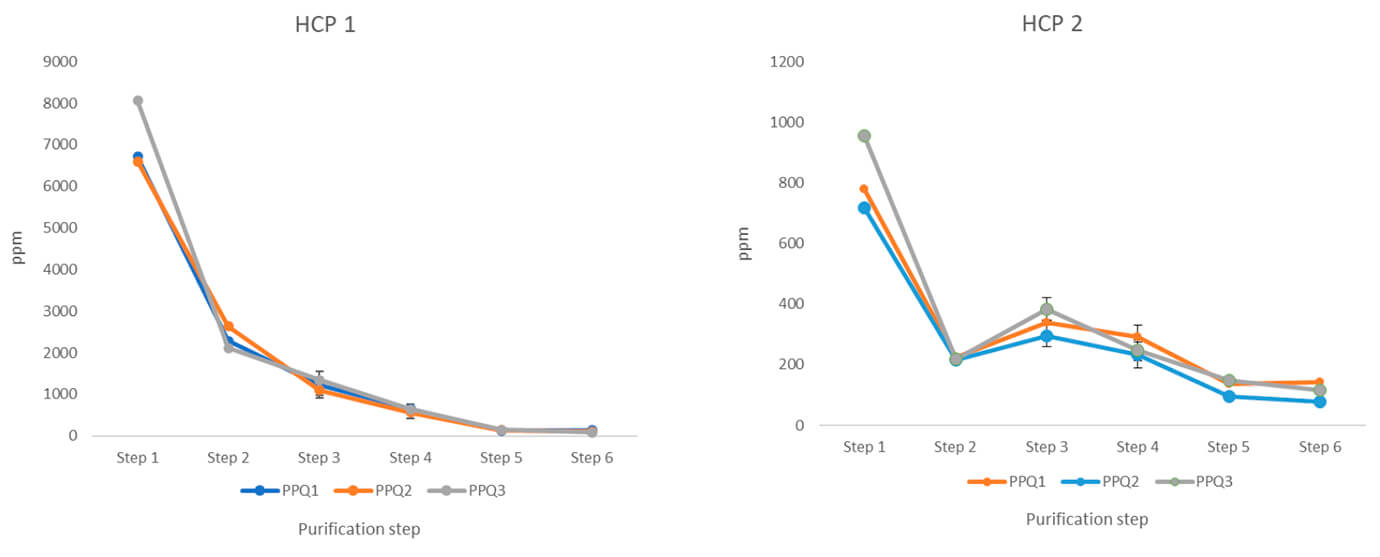
We have also analyzed the process performance qualification (PPQ) runs for HCPs to document process consistency. Our experts made this analysis during the qualification stage of the process validation. The purpose was to evaluate the design of the process and determine if it delivers a quality product – consistently.
In total, the client sent 56 samples for analysis, including three GMP batches from each process step.
- The results included an overview of the number of HCPs and the amount of each HCP throughout the steps. It also compared these results between the three PPQ GMP batches.
- The data formed part of the regulatory documentation for the FDA. They eventually approved the LC-MS data for showing the consistency of the manufacturing process.
A great collaboration for both parties
The ongoing collaboration highly benefits both companies. The client is particularly interested in innovative approaches for protein analysis because they know they will save time and costs in the long run.
Similarly, Alphalyse benefits from their feedback to optimize and further develop sensitive mass spectrometry techniques.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.