Literature
Question:
How do I detect process-related impurities at low ppm levels?
My company develops and produces a biologic. Recently, regulatory authorities asked us to document how efficiently downstream purification reduces specific enzymes added during process development.
How can I measure and follow the levels of enzymes and other proteins added during manufacturing? Is a reproducible method available that detects any process-related impurity down to low ppm levels?
Answer:
Yes, there is a terrific method for process-related impurity detection, with detection limits down to low ppm levels. It can track and quantify the residual protein through all purification steps and, eventually, the final biopharmaceutical product.
However, let’s first look at process-related impurities in process development – and why they increasingly concern FDA/EMA.
The different types of process-related impurities
A process-related impurity is a protein, enzyme, or chemical that follows along with the product during the development and production of biologics, such as monoclonal antibodies, antibody-drug-conjugates, therapeutic proteins, gene therapy products, and vaccines.
Typically, we divide process-related impurities into three subtypes that depend on origin:
- Cell substrate-derived / product-derived (e.g., host cell proteins, host cell DNA)
- Cell culture-derived (e.g., antibiotics, IPTG, DTT, growth factors)
- Downstream-derived / process-derived impurities (e.g., enzymes, buffer components)
Note that the third subtype often includes cell-culture-derived impurities. We also call the third subtype process-related residuals.
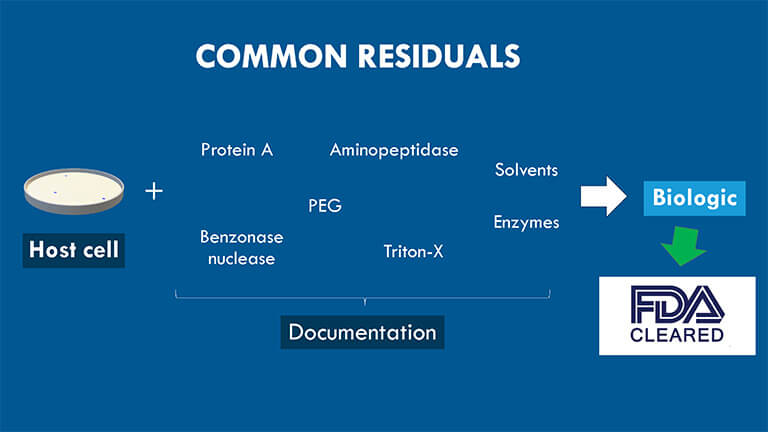
This type consists of agents used to express and purify biological protein products. A standard process-related impurity includes the Benzonase nuclease, enzymes for site-specific PEGylation, aminopeptidase, Protein A, Tris, carriers, ligands, Tween/Polysorbate, DCA, TCEP, heavy metals, solvents, Triton-X, anti-foaming agents, PEI, TFA/Acetate, Imidazole, etc.
Why is a process-related impurity of concern?
Investigating the levels of residuals is crucial, especially in the final product sample. Because, like host cell proteins, residuals can influence the stability and efficacy of the active ingredient, and it may even pose a risk to the patient’s safety [1-3].
Since more sensitive analytical methods exist for residual analysis, FDA and EMA seem increasingly interested in data that map out the purification process’s efficiency [1-3].
How to fully document process-related impurity clearance
FDA/EMA requests an increasing number of biopharmaceutical companies to document residual protein clearance before a biologic can move forward in late-stage clinical trials [1-3].
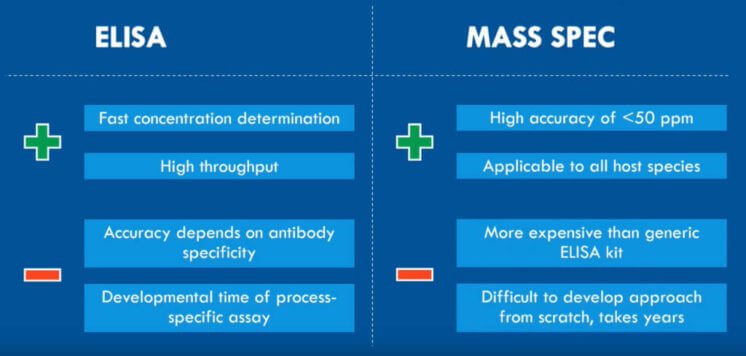
The problem, however, is that older methodologies like ELISA and HPLC are restricted by low antibody specificity and too-high a limit of quantification, respectively. Since the residuals in samples are present in only low-ppm levels, you need a highly sensitive and reproducible approach to get a detailed overview of the residual protein clearance [4, 5].
Luckily, SWATH mass spectrometry (LC-MS/MS) offers precisely that. It fragments all peptides instead of only a group of selected precursors using Data Independent Acquisition (DIA). SWATH LC-MS/MS divides the defined mass range into small mass windows for MS/MS fragmentation of the peptides. Afterward, comparing the acquired MS data to an ion library of known peptides will identify the proteins [6].
The advantages of SWATH LC-MS for residual protein analysis include:
- Highly reproducible identification and quantification due to the use of the DIA mode
- Interference from the high amount of drug substance on the signal of low abundant residuals is kept down by using small mass windows for MS/MS fragmentation.
- Specific residuals can be followed throughout the entire process development. You can also use it to compare batches and for quality control after upscaling.
- A process-specific assay can be developed within just a few weeks.
- High sensitivity makes it possible to quantify low ppm levels.
With these advantages, SWATH LC-MS is ideal for analyzing process-related impurities in biologics development [6].
Need more information on the analysis of process-related impurity?
Check out a blog post about the SWATH methodology or click here for more details about Alphalyse’s process-related impurities analysis.
References
[1] U.S. Department of Health and Human Services – Food and Drug Administration: “Immunogenicity Testing of Therapeutic Protein Products — Developing and Validating Assays for Anti-Drug Antibody Detection: Guidance for Industry,” 2019
[2] U.S. Department of Health and Human Services – Food and Drug Administration: “Q3C Impurities – Residual Solvents: Guidance for Industry“, 1997
[3] European Medicines Agency: “Guideline on Immunogenicity assessment of therapeutic proteins,” 2017
[4] Zhu-Shimoni et al.: “Host Cell Protein Testing by ELISAs and the Use of Orthogonal Methods,” Biotechnology and Bioengineering, 2014
[5] Bracewell et al.: “The Future of Host Cell Protein (HCP) Identification During Process Development and Manufacturing Linked to a Risk-Based Management for Their Control,” Biotechnology and Bioengineering, 2015
[6] Heissel et al.: “Evaluation of spectral libraries and sample preparation for DIA-LC-MS analysis of host cell proteins: A case study of a bacterially expressed recombinant biopharmaceutical protein,” Protein Expression and Purification, 2018
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.
