Literature
Why measure process-related impurities in gene therapies with LC-MS?
December 2 2020, by Ejvind Mørtz

Question:
Can I use mass spectrometry to document process-related impurities?
I work in a biopharmaceutical company developing a gene therapy product based on an adenovirus vector. Recently, I have become responsible for setting up analytical methods, including impurity assays. However, it is difficult to find suitable methods since there are no good commercial HCP ELISA kits for our human cell line.
So, can I use a method like mass spectrometry to document the process-related impurities?
Answer:
To put it simply: Yes, absolutely.
But let me explain further why mass spectrometry is a competent analysis method for protein impurities in cell and gene therapy products.
Research in the regenerative therapy field has advanced dramatically in the last few years. In 2018, FDA received 206 Investigational New Drug (IND) submissions, and FDA is expected to approve 10-20 cell and gene therapy products (GTPs) per year by 2025 [1, 2]. So, in response to these many new gene therapies and medicinal products (GTMPs) in development, FDA and EMA recently published new guidelines which advise developers to monitor process-related impurities [3, 4].
The manufacturing processes of GTPs are usually quite complex, and only small batches are produced for small patient populations [5]. The process uses new cell lines and ads various proteins from multiple sources and organisms during cell growth and harvest. These include bovine serum, human albumin, cytokines, antibodies, benzonase, etc.
Thus, a standard commercial ELISA cannot measure all these impurities. Also, it would be costly and challenging to develop a product-specific ELISA to cover the HCPs in these products. Luckily, mass spectrometry methods provide a more suitable analysis for GTPs.
(You can read more about the pitfalls of HCP ELISAs in this blog post.)
Now, let us have a closer look at the nature of cell and gene therapies
Cell therapy (also known as cellular therapy or cell transplantation) products contain a form of viable cells, e.g., CAR T-cells. These can come from a donor person (allogeneic cell therapy) or the patient himself (autologous cell therapy). They may even come from another species (xenogeneic cell therapy).
The cell types are, for instance, stem, progenitor, or primary cells. Often, the therapies are designed for neurological, autoimmune, cardiovascular, or ophthalmologic disorders [6, 7].
Gene therapy medicinal products (GTMPs or GTPs) consist of a carrier vector or delivery system. They typically contain a nucleic acid sequence, a virus, or a cell and classify as genetically modified organisms (GMOs).
The target can be specific tissues or cells to regulate, repair, replace, add, or delete a genetic sequence and affect a particular type of protein or class [6].
The two major GTP classes are recombinant viruses (viral vectors) and nonviral methods (e.g., plasmid DNA or bacterial vectors). The viruses carry human DNA to replace disease-causing genes in the patient. Retrovirus, adenovirus, herpes simplex, vaccinia, and adeno-associated virus (AAV) are highly effective and thus widely applied. However, nonviral methods may carry a lower risk of patient immunogenicity [8].
LC-MS – An orthogonal method for analysis of residual protein impurities in gene therapies based on adenovirus
So, let us talk about how you analyze protein residuals in products based on adenovirus expressed in a human cell line, e.g., HEK293 or A549 cells, and grown on a cell substrate containing serum albumin.
Adenoviruses comprise a protein capsid with different proteins enclosing the DNA and core proteins (See the figure below). When the virus is grown and purified from the human cell, the virus drug substance samples will also contain small amounts of residual human proteins. Thus, the high number of different proteins in the drug requires characterization by a highly sensitive approach before clinical administration [9-11].
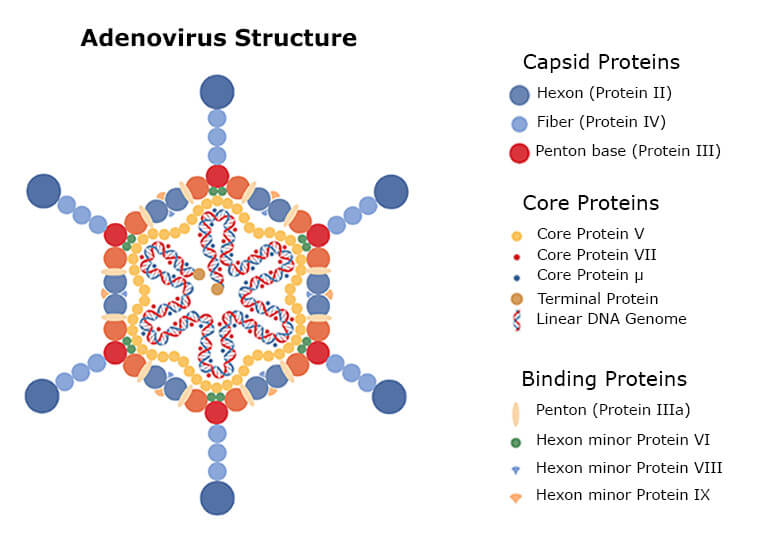
The best analysis method for this mixture is LC-MS, as it can both quantify and identify the different protein impurities. The SWATH LC-MS (liquid chromatography – mass spectrometry) analysis is a highly reproducible residual protein and HCP analysis method.
The SWATH LC-MS analysis provides A) The total amount of residual host cell proteins in ng/ml, B) A list of identified residual proteins and their amounts, and C) A list of identified viral proteins and their amounts.
Since the analysis is based on peptide analysis by LC-MS/MS, it does not rely on animal immune responses and can identify and quantify individual proteins – it is ideally suited for documenting process-related impurities in the complex GTP biopharmaceuticals [12, 13].
FDA guidelines for documentation of impurities in cell and gene therapies
Hopefully, you now have a basic understanding of the impurity analysis of GTPs. If you would like to learn more about how to apply mass spectrometry for impurity analysis of cell and gene therapy products, I recommend you read one of these articles:
- BioPharm International: A Novel Method for Host Cell Protein Analysis
- Biopharma from Technology Networks: A Smarter Way To Remove Host Cell Protein Contamination From Gene Therapies
Also, I have recorded a short webinar on FDA guidelines for monitoring impurities in cell and gene therapies:
References
[1] Lukashev et al.: ”Viral Vectors for Gene Therapy: Current State and Clinical Perspectives.”, Biochemistry (Moscow), 2016
[2] Hernandez Bort et al.: ”Challenges in the Downstream Process of Gene Therapy Products,” American Pharmaceutical Review, 2019
[3] European Medicines Agency: “Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products,” 2018
[4] U.S. Food & Drug Administration: “Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs),” 2020
[5] Mavilio, F.: “Gene therapies need new development models,” Nature, 2012
[6] Buzhor et al.: “Cell-based therapy approaches: the hope for incurable diseases,” Regenerative Medicine, 2014
[8] Nayerossadat et al.: “Viral and nonviral delivery systems for gene delivery,” Advanced Biomedical Research, 2012
[9] Krawitz et al.: “Characterization of Residual Host Cell Protein Impurities in Biotherapeutics,” Analytical Characterization of Biotherapeutics, 2017
[10] Rux et al.: “Adenovirus structure.”, Human Gene Therapy, 2005
[11] Jin et al.: “Direct Liquid Chromatography/Mass Spectrometry Analysis for Complete Characterization of Recombinant Adeno-Associated Virus Capsid Proteins.” Human Gene Therapy Methods, 2017
[12] Wohlrab et al.: “Tracking Host Cell Proteins During Biopharmaceutical Manufacturing: Advanced Methodologies to Ensure High Product Quality,” American Pharmaceutical Review, 2018
[13] Goey et al.: “Host cell protein removal from biopharmaceutical preparations: Towards the implementation of quality by design.”, Biotechnology Advances, 2018
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.