ELISA reagent characterization and HCP coverage analysis
When you need a detailed characterization of your HCP-ELISA antibodies, advanced ELISA-MS™ is the best technology available. Unlike other methods, ELISA-MS™ analysis utilizes immunocapture directly in the ELISA well and measures the actual protein-antibody binding using LC-MS without artificial systems, thus mimicking the actual conditions of the ELISA for evaluating the HCP coverage.
With ELISA-MS™, you can accurately evaluate the quality of antibodies in any commercial, platform, and process-specific kit and identify the individual Host Cell Proteins (HCPs) measured by the ELISA antibodies - and also the HCPs that are not measured.
This detailed characterization of ELISA antibodies enables you to evaluate and select the best-fit ELISA for monitoring the product- or process-related impurities in your product. It also reduces the risk that abundant or potentially problematic HCPs go undetected by your ELISA.
Many of our clients have successfully used ELISA-MS™ data to document the suitability of commercial and process-specific assays, including it as part of IND and BLA submissions.
Why use LC-MS for ELISA characterization?
Compare ELISAs and document HCP coverage
Document coverage percentage, identify covered vs. uncovered HCPs, and get a list of high-risk HCPs – enabling you to select the best fit ELISA for your product
Bridging studies
Characterize ELISA reagents and HCP standards to assess why the ELISA shows dilutional nonlinearity or inconsistent results
Troubleshooting ELISAs
Investigate issues caused by antibody/ drug substance cross-reactivity, insufficient specificity, incorrect standards for the calibration curve, etc.
Many of our clients face the challenge that different ELISA kits, even from the same vendor, can show very different results — sometimes more than 10 to 100-fold in- or decreases in HCP amounts — when analyzing identical batches.
Using LC-MS, we can uncover if the inconsistent results may be due to an unintended change in the HCP standard. Such a change can make it a poor fit for your drug sample or antibodies, resulting in a different HCP concentration readout even with the same HCP antibodies. Other reasons for the inconsistent results, which ELISA-MS™ can uncover, might be that a new generation of antibodies binds better — or worse — to the proteins in your sample.
Regulatory authorities are concerned that abundant HCPs in a drug product may go undetected by ELISA or that your drug substance may contain undetected HCPs of concern that impact patient safety or drug stability.
ELISA-MS™ data provides not only the coverage percentage of your ELISA but also a list of the exact HCPs recognized by your ELISA antibodies. In addition, we provide access to our extensive database of HCPs of concern – based on current regulatory guidelines, literature, analyses of commercially released products, and our experience from more than 500 client projects – allowing data-driven risk assessment of the HCPs in your product. This is solid documentation for regulatory authorities.
Not all commercial HCP ELISAs may be equally suitable for detecting the specific HCPs in your biologic. Comparing the coverage of various ELISAs — commercial kits or process-specific assays — allows you to pick the best and most fitting ELISA for your drug and process-related impurities. Furthermore, you must ensure that the ELISA standard resembles the HCPs in your drug substance, or the readout may be off.
ELISA-MS™ combines ELISA-based immunocapture with LC-MS analysis to investigate which ELISA antibodies bind to your early process sample. The result is a very accurate and detailed list of individual HCPs in the sample recognized by the ELISA antibodies. You can then compare and base your choice of kit not only on the total coverage but also on each ELISA’s ability to detect individual HCPs, e.g. HCPs of concern. Furthermore, LC-MS can be used to analyze the fit of the ELISA standard to your drug substance.
Typical project process
You typically work with
these experts:



Project scope
We like to start with an online meeting to learn more about your project. Based on your needs, you will receive a draft proposal outlining the suggested analyses and expected timeframe.
Samples
After signing the final project proposal, we will contact you for details about your samples. An estimated report delivery date will be sent as soon as we receive your samples.
Execution
An Alphalyse project leader will oversee the project and send you status updates by email regularly.
A typical analysis includes:
- ELISA-MS™ analysis of selected HCP antibodies using a harvest sample.
- LC-MS-based HCP analysis of harvest, mock, and/or drug substance (DS) samples.
Results
You will receive the analysis report by email. Depending on the project, it includes:
- Objectives, description of analytical procedure, results, and conclusions.
- Reporting of HCPs covered by the ELISA antibodies in the sample and their distribution, total HCP coverage percentage.
- Evaluation and comparison of HCP profiles of the samples and/or to the ELISA standard.
- HCP ELISA potential high responder HCPs.
- HCP antibody binding of HCPs of potential concern (CHO projects only).
- Selected raw data.
Follow up
Upon project completion, your team is invited to go over the results at an online meeting.
Curious to know more about HCP coverage analysis?

Whatever ELISA-related challenge or question you may have, we are here to help you solve it. One of our protein analysis experts will discuss the best analysis approach or method for your project by email or online meeting – without obligation.
Client stories
Blog: Why results obtained by new ELISA kit differ from the original
Troubleshooting HCP ELISA results using LC-MS and ELISA-MS™
Characterization of a mock sample before ELISA development
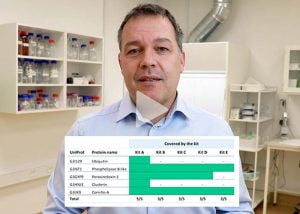
Selecting the best HCP-ELISA kit out of five
Video: Trouble-shooting ELISA results using mass spectrometry
HCP-ELISA coverage analysis without a null cell line
Video: Selecting the best suited HCP-ELISA
Video: HCP coverage analysis by ELISA-MS
Video: Problems with non-dilutional linearity of ELISA
Ebook: Comparison of HCP ELISA coverage methods
Webinar: 3 reasons to check the coverage of your HCP-ELISA
ELISA characterization and HCP coverage analysis
Fast assay setup and analysis – 8-12 weeks.
No null cell line is necessary.
Requires only a low amount of antibody.
Applicable for all types of biologics: Monoclonal antibodies, recombinant proteins, gene therapy, vaccines, etc.
Suitable for all commercial, custom, process- and platform-specific ELISAs.
Tested on commercial ELISAs from all major vendors.
What clients say
Knowledge center
How do the measured MS HCP levels compare to measured ELISA HCP levels?
The principles of ELISAs and LC-MS are inherently different. However, in our experience, a fit-for-purpose ELISA with good coverage of relevant HCPs performs comparably to LC-MS analysis. However, if the ELISA is unsuitable for the product, e.g., a generic ELISA with low coverage of HCPs in the specific process, the measured amounts of HCPs might be entirely off in the ELISA.
ELISA-MS™ analysis combines immunocapture with mass spectrometry and can determine if your HCP-ELISA is fit-for-purpose and capable of accurately detecting the HCPs of concern in your product.
How is ELISA-MS™ performed – and what makes it different from other ELISA coverage analyses?
The Alphalyse ELISA-MS™ analysis is unique in utilizing immunocapture directly in the ELISA well combined with LC-MS to identify the proteins. We do not use artificial systems, e.g., affinity chromatography columns with repetitive loading of samples, for extracting the maximum number of proteins and artificially increasing the coverage percentage, as we believe results achieved this way do not reflect the actual binding of HCPs to ELISA antibodies in praxis and risk inflating the coverage percentage.
While doing immunocapture directly in the ELISA well may yield slightly lower coverage numbers (20-80%), we believe these numbers accurately reflect the efficiency of your ELISA in monitoring HCP levels in your samples.
To perform ELISA-MS™, an early process sample containing the drug substance is used for analysis, eliminating the need to develop a null cell lysate from a mock fermentation. The Host Cell Proteins in the sample are immunocaptured using the ELISA antibodies and identified by LC-MS/MS. This generates a comprehensive list of the specific HCPs recognized by the ELISA antibodies.
By comparing this list with all the proteins found in the early process sample, we can evaluate the coverage of your ELISA. Additional HCP analysis of your drug substance (DS) allows a comparison of the HCPs covered by the ELISA to the HCPs found in your DS.
How do you ensure digesting only the antigen and not the antibodies in the ELISA-MS analysis?
After adding the antigen, we perform a gentle protein/antigen digestion directly in the ELISA plate. This procedure leaves the ELISA antibodies largely intact as they are somewhat resistant to the proteases, unlike the HCPs. In addition, we check the antibody sequences against our extensive protein database to eliminate any antibody peptides generated by the sample preparation.
Ebook:
Videos:
- Characterization of ELISA reagents: Mock and harvest HCP comparison
- Characterization of ELISA standard: Trouble-shooting ELISA
- Coverage analysis using immunocapture and LC-MS: Orthogonal HCP method for vaccine development and production
- HCP coverage comparison using ELISA-MS: Selecting the best suited HCP-ELISA
- Non-dilutional linearity of ELISA: Hitchhiker HCPs
- HCP-ELISA coverage analysis without a null cell line
Client cases:
- Selecting the best HCP-ELISA of 5 kits
- Characterization of an ELISA standard: Comparing HCPs in mock and harvest samples
- Troubleshooting HCP ELISA results using LC-MS and ELISA-MS™
Blog posts:
- How do ‘Jackpot HCPs’ and a ‘Lucky Goat’ influence my ELISA results?
- Can I use commercial HCP ELISA kits in clinical trial documentation?
Research articles:
- A novel approach to evaluate ELISA antibody coverage of host cell proteins
- Monitoring process-related impurities in biologics - host cell protein analysis
Poster:
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.